Copper is among the most important metals and has been in use since the beginning of civilizations. Copper is obtained from ores found in deposits spread around the world. Canada has been a major producer of copper for a long time and still has some billion dollar companies such as Solaris Resources inc. and Lundin Mining Corp. focusing on copper mining. The process of obtaining the metal from its ore requires high temperatures and extreme care.
Extracting Copper
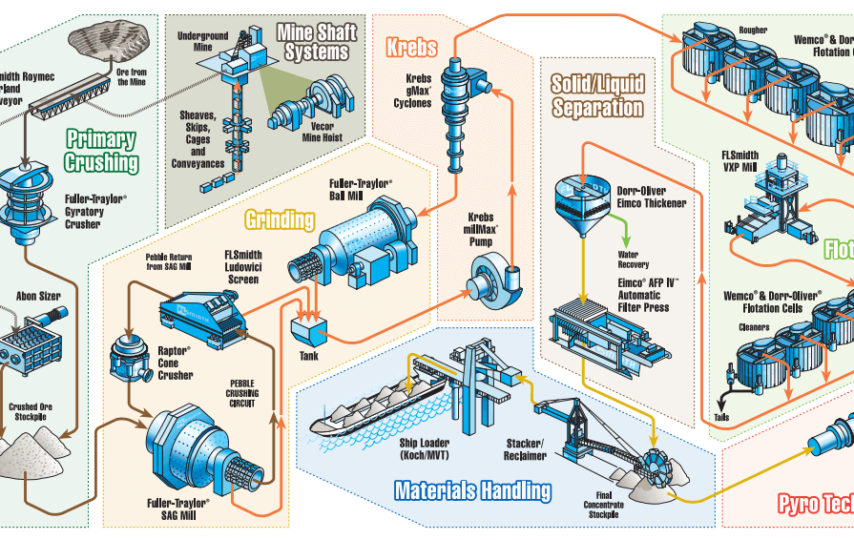
Copper occurs in sedimentary and igneous rocks and is present in high amounts in the earth’s crust. However, some places are extremely rich in compounds of copper. These minerals, rich in copper content, are called its ore. The extraction is briefly explained below.
1. Grinding – As per the engineers at Canadian copper mining companies, once the copper ore is extracted, it is crushed to make powder.
2. Concentrating – Crushed ore is to concentrate the sulfide ore by mixing with the oil. This process is called froth floatation, and the unwanted material called gangue accumulates in the tank and is later removed.
3. Roasting – Powdered ore is roasted using heated air to get rid of sulfur.
4. Smelting – Flux is added to the ore in tanks called thickeners and then sent to smelting furnace where it gets heated up to 2300 ֯F. The ore is now in liquid form, which is poured into a slag-settling furnace. The slag settling furnace forms a mixture of copper, sulfur, iron, and slag. This mixture is about 60% copper.
5. Matte Conversion – According to the engineers at Solaris Resources inc, the matte is then poured into the converter. The mixture is purified to a yellow form of copper called blister copper, which is 98% pure.
6. Anode Casting – Blister copper is burned in an anode smelter, and it turns blue-green. This form is 99% pure and is used to make copper anodes. Once cooled, it turns cupric in color.
7. Refining using electrolyte – At this stage, copper is purified to 99.9%. The process occurs in a giant electrolytic cell. The electrolyte is a mixture of copper sulfate and sulphuric acid. The anode is a slab of impure copper, while the cathode is made of a thin sheet of pure copper. The current is passed through the electrolyte, and cations from the anode travel to the cathode, and other impurities settle down in the tank. It takes about two weeks for electrolysis to stop when the anode disappears, and the cathode is 99.99%, pure copper.
The giant slabs of copper are then washed and rinsed to remove the electrolyte. The metal is then sent to factories to be made into wires, pipes, utensils, and other products.
The impurities produced in the electrolysis are further refined for precious metals like gold and silver.
Conclusion
Copper Mining is a wide industry, and due to the increasing electric network and usage of copper pipes, it is continuously growing. Copper mining is being done at a rapid pace. The process is a combination of pyrometallurgy and electrolysis.